Kossel-Lewis Approach to Chemical Bonding
Kossel-Lewis Approach to Chemical Bonding: Overview
This Topic covers sub-topics such as Atom, Chemical Bonds, Covalent Bond, Lewis Acids, Coordinate Bond, Valence Electrons, Lewis Dot Structure, Formal Charge, Types of Chemical Bonds, Chemical Compounds, Double Covalent Bond and, Triple Covalent Bond
Important Questions on Kossel-Lewis Approach to Chemical Bonding
Draw the structure of tetra cyano ethylene.
Calculate the number of lone pairs and bond pairs in molecule.
Write Lewis dot structure of carbon tetrachloride.
Why an element with electrons in the outer most shell is stable?
Find the formal charge of .
An element has protons and its valency is . Another element has valency . What is the difference in the number of electrons in ?
Which of the following statements is true?
Lewis dot structure of .
What type of bonding is there between hydrogen and fluorine in molecule ?
According to Lewis structure the number of lone pair & bond pair of electrons in ion.
When reacts with then after the reaction
Write Electron dot structure of Sodium
What is the dot structure of HMnO₄
Give an example of a covalent bond formed by dissimilar atoms.
Which compound has both covalent as well as co-ordinate bonds?
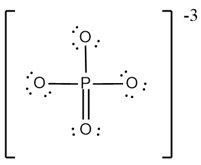
What are the formal charges on and in the Lewis (electron dot) structure of the phosphate oxyanion represented in the figure?
Identify the name of the noble gas compound formed by the following reaction:
Write the reaction for the formation of xenon difluoride.
Give 2 drawbacks of Kossel's Postulate.
Define group valency. What are the valencies of all the groups in the periodic table?
